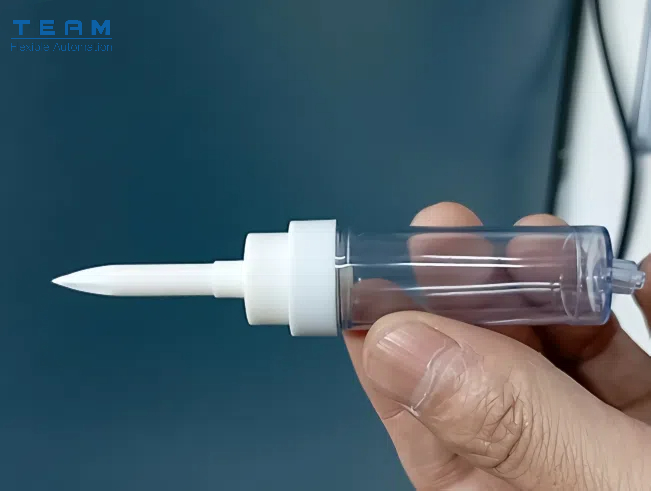
Medical products, especially injection-molded medical devices, have two significant characteristics: high sterile requirements and a wide variety of products with clear production peak and valley fluctuations.
Sterility Requirements in Medical Products: Sterility cannot solely rely on the disinfection process; products must also maintain a good surface roughness.
Let’s conduct a simple experiment. If you draw a line with a marker on paper with different roughness levels, a smoother surface allows the mark to be easily erased.
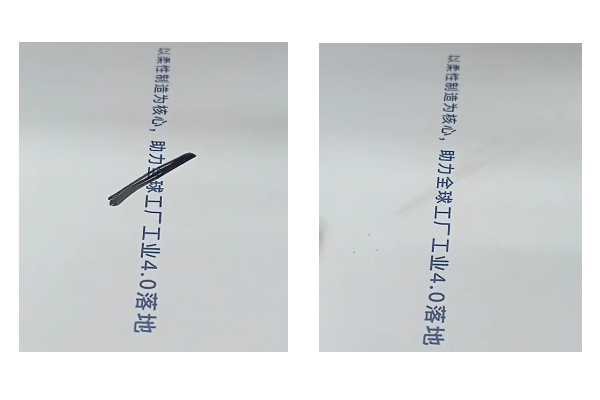
However, a rougher surface traps dirt and grime, creating ideal conditions for bacteria to thrive.

Thus, when automatically assembling these products—especially during the feeding stage—it’s crucial to consider whether the feeding method could pose a risk of scratching or damaging the parts.
Flexible Feeding in Medical Injection Molding Assembly
For medical two-part assembly equipment, the use of flexible vibration feeding is not just for compatibility, but also because this method, although less efficient than other techniques, minimizes the damage to the surface roughness of injection-molded parts. Flexible vibration trays move with low amplitude up-and-down vibrations, without the need for long-distance sliding friction. Rubber protective covers can be added to the grippers to further protect the surface of the products.

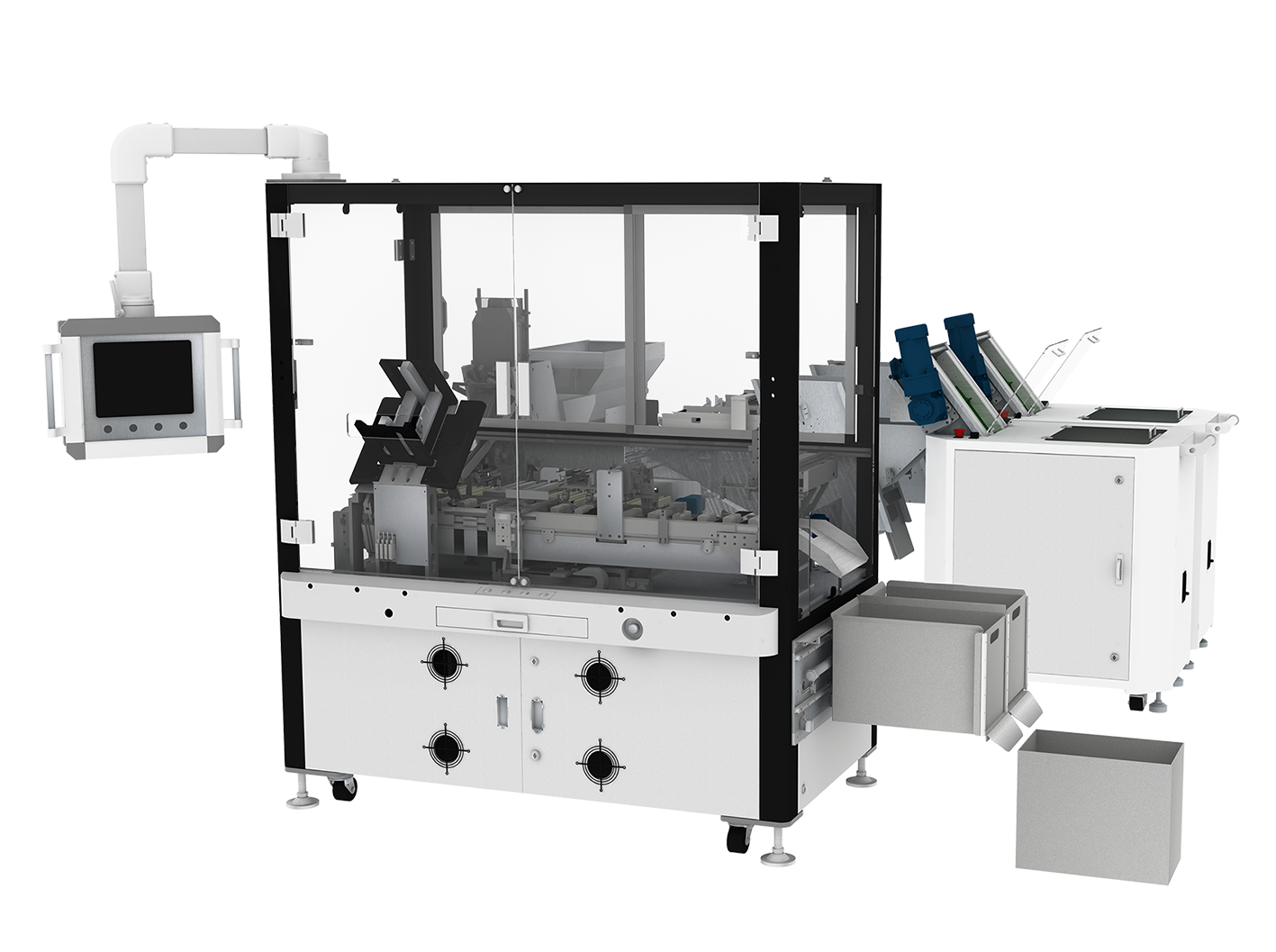
Standardized Assembly Modules for Flexible Production
During the assembly stage, we have created standardized modules for common assembly actions such as screwing, insertion, gluing, sealing ring installation, and rubber band insertion. These modules are capable of flexible production within a certain stroke range, achieving the following requirements:
1. Same Assembly Action, Same Product Type, Different Specifications – Easily compatible.
2. Same Assembly Action, Different Products – Only partial fixture replacements are needed within a certain size range.
3. Different Assembly Actions, Different Products – Quick tool change allows for fast conversion to different production setups.

Standardized assembly modules, tested and iterated by the market, provide more stable performance compared to non-standard modules. During maintenance, issues are easier to pinpoint, and spare parts can be processed more quickly.
Interestingly, when a company has several dual-part flexible assembly units, they can quickly adjust production capacity by swapping different fixtures. For example, if a customer experiences a sudden surge in demand for product B, all the machines can switch to product B's assembly modules and start production immediately. This is something that would be impossible in the era of dedicated machines. Facing production fluctuations, companies either had to add more machines or shut down production lines, which was a very passive approach.

Automation and the Evolution of Standardization
As automation equipment evolves from custom-built (non-standard) to increasingly standardized systems, it’s not enough to just improve the machinery and control systems. A deeper understanding of the production process and associated risks is essential to ensure that the results are not just “toys,” but high-quality, reliable medical products.











 Home
Home Products
Products Telephone
Telephone Message
Message



